9:30 am – 10:45 pm
To do
- submit progress report to DR. (done)
- email Dell about RAM (done. Dell is very slow with reply. Probably should just go Amazon)
- email DA about letter (with CV) (done)
- work on slides (postponed)
- work on DF application (postponed)
Embryo fixation
- flip plates, 9:15 am.
- flip plates, 1:45 pm.
- Fix embryos 6:45 pm (6 – 9.5 hrs)
Fly RNAi
- PCR cleanups (done).
- order qPCR primers
- order qPCR kit (done)
- set up T7 reactions (done)
- clean up dsRNA
- out of columns
- washed used columns with water and requilibrated with RNA binding buffer
- this protocol is much slower — maybe I want to go back to magnetic beads…
- set up RNAi experiments (can’t do)
- run gels for 8 new samples (postponed)
RNA synthesis purification failed
- attempted on-column DNase treatment following Zymo instructions (in ~70% ethanol, this is a stupid idea).
- next time just add DNase to the in vitro mix at the end of the in vitro reaction.
- samples seem to have degraded and run very funny on agarose borax gel.
- testing now pre and post column purification treated RNA on a TBE acrylimide gel.
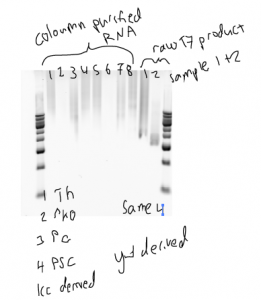
- borax doesn’t run RNA very well, looks much better on a PAGE gel (though still awfully smeary).
- still — T7 seems to have performed very badly, quite smeary and low yield (50-200 ng/uL in 120 uL elutions).
- oddly sample 2 — weakest band, least smeary, has easily the highest concentration (~800 ng/uL)
- not sure what this ‘trapped in the well’ phenomena is either…

Start again!
- set up PCRs to amplify targets from genomic DNA and add T7
- running PCR overnight.
