10:15 A – 6:50 P, 7:50P – 10:30P
Probe making
- have not confirmed nicking enzyme concentration for digestion. Canceled PAGE introduction with Bryan, will attempt again later.
Telomeres
- Read over telomere manuscript from John Wu
- Imaging telomeres: 656 = 60 mW, 405 = 1.2 mW (don’t need all of it though). 20,000 frames.
- Find region, shoot test-storm movie to get power parameters.
- Image in 3D (generally not using 3D info in later analysis though).
- Steve on STORM3 has serious issues in tiling errors (plots new field of view ontop of previous position about every 3rd frame leaving one blank gap and one misplaced image.
Cell staining
- Rinse out secondary
- post-fix
- mount for STORM
- 647 not swtiching well at all for STORM. Laser power ~135 mW, reasonably strong conventional signal. difficult to seal slides well.
- with 3 layers of tape and 15o uL of fresh buffer still fairly poor photo-switching / high background. Sample also not very flattened, most labeled centromeres are not near coverglass.
- 488 extremely bright. Have to bleach and STORM to see lamina and not just glowing signal from cells. Too much out of focus light I think to get good optical sectioning on conventional images.
- slide 1 very weak sparse foci in 750, 4-8 per cell (H3K9me2?) not strongly co-localized with centromere foci but hard to tell.
- Slide 3 completely clean of 750 signal.
- Slide 2 (Pc) also poor switching. Clear 647 signal in conventional, judging by relative brightness on turnkey though not so good.
- Might all be an issue of too dense cells / not happy and not staining well. Films of cells where still stuck very bright in all labeled channels, not imagable, too dense, too much background.
- OR I bet they overfixed to be able to react with antibodies…
- Start new cell staining in chambered slides (had better luck imaging. Will leave lid off to avoid whicking of solution mixing samples).
- Try labeling antibodies first (4C O/N), short 5 min post fix, then prep for D-FISH.
Snail
- Testing Espl amplifications (2o min extension time using NEB LongAMP taq, 50s/kb)
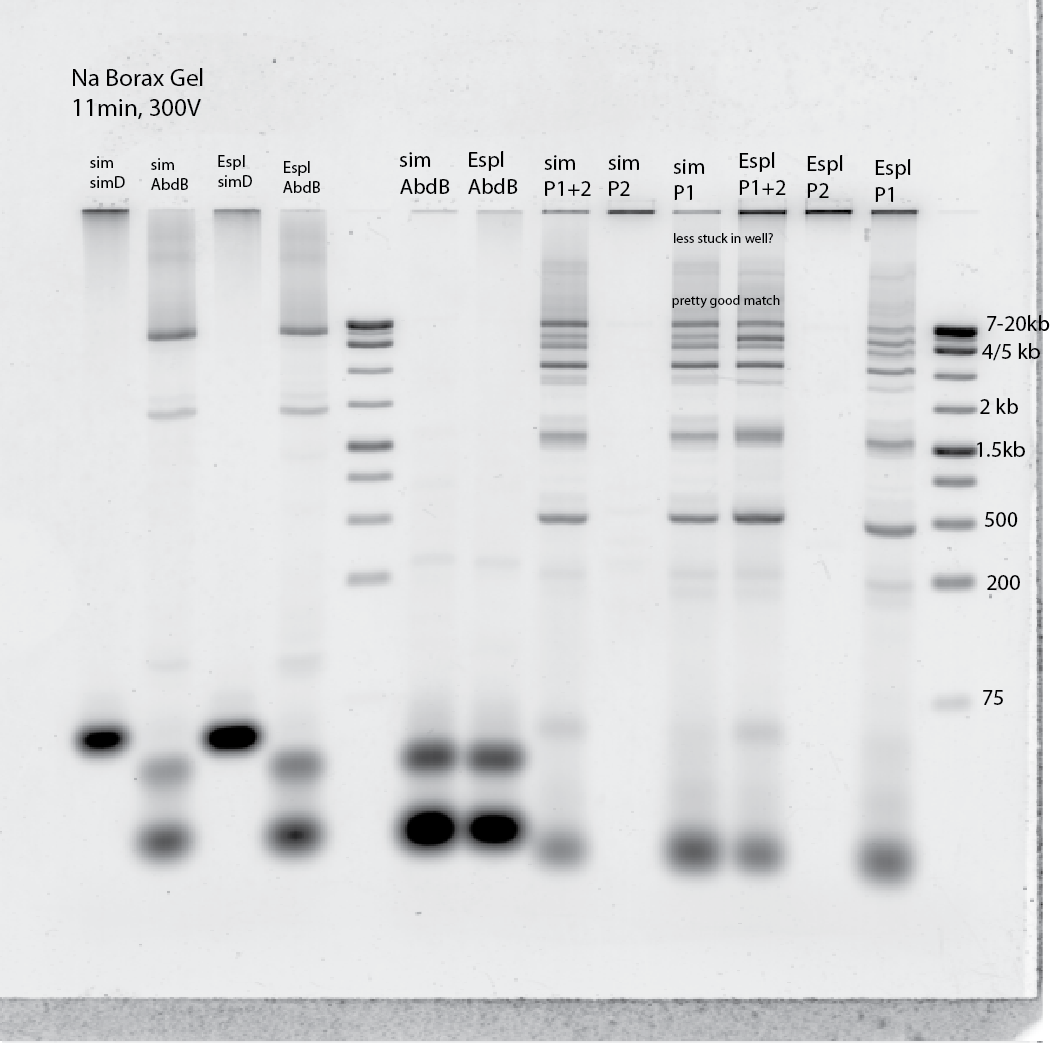
- Run on TAE gel to separate the 20 kb stuff better, Borax not much resolution above 4 kb
- # Try higher annealing temp (65 instead of 60C) and longer extension (25 min). Also change to a different control primer?
- # Design new sim[D] and E(spl) probes
Coding
- Useful matlab function on findcircles
- Some quality improvements in Matlab R2012b
write to Francis about Bender notes + SCM
# Send letters to more MERCK people. (should probably do phone calls)…
