9:00 A – 10:00 P
Embryo collection
- Flipped Esc cage — good looking overnight collection
Probe design
- schematic of probes
- design and send to Brian new probe sequences for Custom Array order.
- working on new probe sets for colors project
- wrote simple script to screen out short regions of other chromatin types to allow for bigger almost contiguous section classification.
-
Wrote script to intersect regions (fast and memory efficient approach, no loops over chromatin indices), allowing for a combined red+yellow = orange classifier.
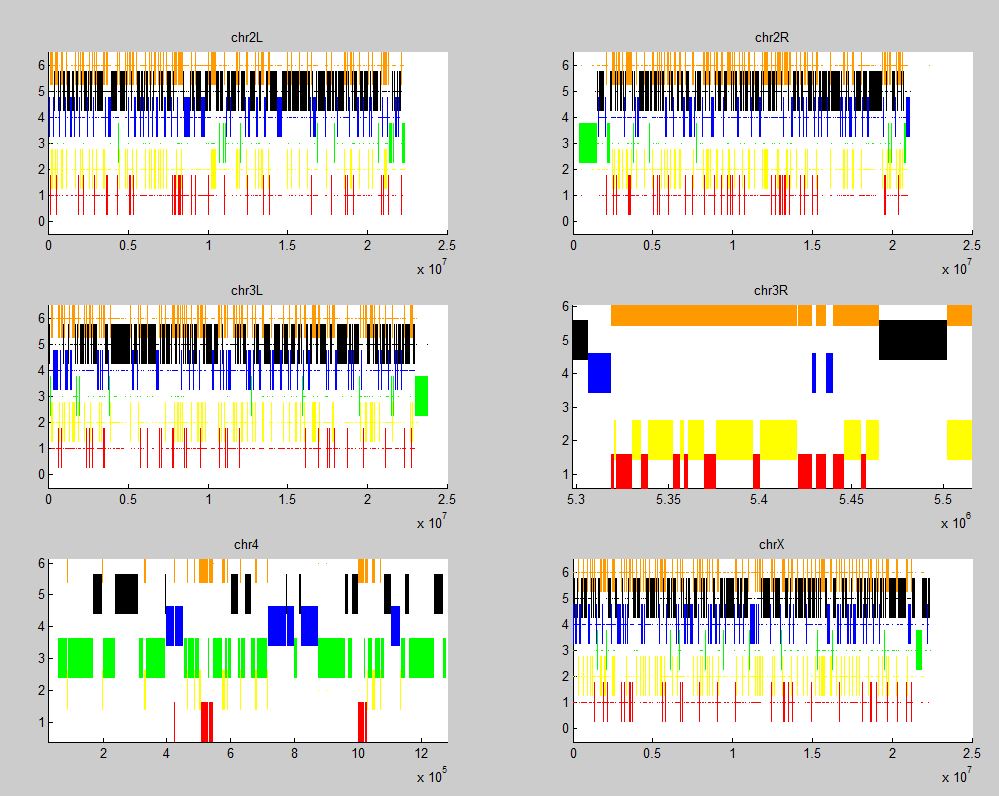
-
still need to modify script to ensure region has adequate fractional representation of the primary type — not just tiny stripes breaking up maximal sized ‘min gaps’. e.g. this red/yellow region downstream of BX-C still get classified as part of the extended blue region from the BXC:
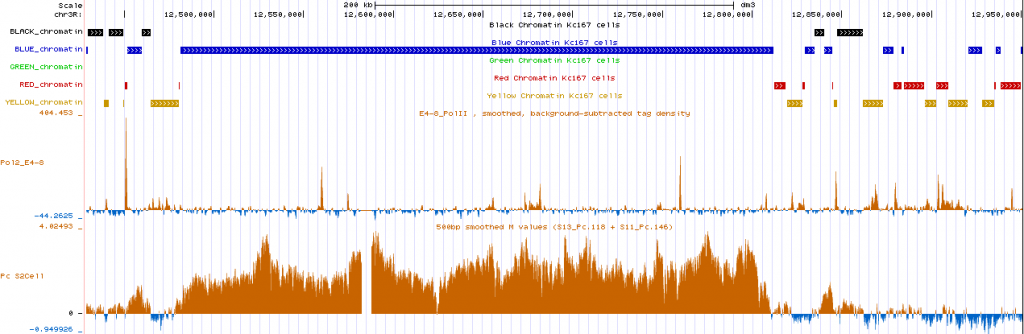
Review
- working on reviewing paper
Embryo Ucryo section staining.
- Documenting revised coverglass staining protocol
- washing off dual-O/N primary secondary at RT in PBT coplin jar (keep slide hydrated)
- Post-fix with 4% PFA .1% GA at RT for 5 min on coverslip mount in moisture box. Don’t aspirate off PBT just give quick shake onto kimwipe and add 400 uL fix mix on top.
- submerge fix-solution + coverglass into petri dish with PBT to rinse. Move to PBT coplin jar 10 min.
- Heating 2x SSCT 50% formamide to 92C in petri dish on heat-block set to 94C. (small mount inside petri dish to facilitate easy retrieval of coverglass).
- Heating 2x SSCT 50% formamide to 60C in small incubator.
- Move sample to 2x SSCT 10 min.
- Move sample to 2x SSCT in 50% form 5 min.
- Incubate sample 2.5 min in 92C(?) pre-hybe bath
- Incubate sample in coplin jar at 60C, 20 min.
- Measured heat-bath temperature with thermometer — noticeable gradient in small petri dish. Thermometer reading around 50C. Gets up to 55C at max temp setting of 120C on block. Should use waterbath downstairs.
- Cleaned downstairs waterbath. Added clearbath solution.
- solution on coverglass still boils at 118C heatblock. Try heating glass coverslip up to boiling and find 100C point at glass surface. — not looking like a good way for calibration.
- NEW: petri dish on hot block in waterbath, filled so that sides of petri dish are contacting water. Fill dish with pre-hybe solution and place slide in that. (Or just submerse the coplin jar in the 92C heat bath). Denaturing the slide still do on heat block in air with a little bit of water between glass slide and metal block.
- Oven does not drop from 60C to 42C very fast. Keeping door open to push it to 42C and then closing and it heats right back up to 66C (must have memory of rate of temp change and be trying to keep it stead). Tried turning it off, cooling down to ~40C and turning on again.
- Chamber still holding at 46C and heating, despite 42C set, 30 min later. :(.
- Check slide on Turnkey, 8P.
FAILED.
- between issues with incubators / baths and the short incubation, the PAINT seems to have failed. Maybe it doesn’t like the post-fix.
- No sign of AbdB staining either, though the background in yolk vacuoles is quite high in this channel, which I’m pretty sure is specific to having the antibody incubation (or at least the GA treatment).
- no crazy backgound on coverglass noticeable, and no loss of sections, DAPI still looks great. (sections still could be much flatter). At least the keep hydrated approach seems to help there.
- slide currently in PBT. Should keep to double check no background on STORM scope.
- Try again tomorrow, straight DFISH first, short antibody treatment second if DFISH works, the image. Just like the successful BXC-paint + 488-H3K27.
