10:00A – 7:45P, 8:30P – 11:15P
Project 2
OligoPaints
More troubleshooting diagnostics
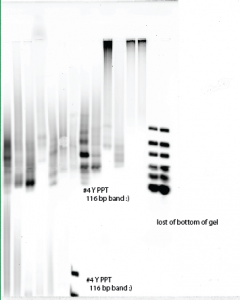
- Gel order
- #2 final, #3 final, y10mL DE, B10ml DE, #6 PCR, # 7 PCR, #9 PCR, #8 PCR, #4 EP1, #5 EP1, y-assym column, b-assym column, BP T7, BPC T7, my ULR 2uL, gel bench ULR 2uL.
- lower rows: (#1 final) #1 no-glyc, #2 no glyc, #3 no glyc, #4 no glyc # 5 no glyc, #4 ?, ladder.
- 300 V, 14 min, 2% ultra-pure agarose gel in Borax.
Agarose gel 2 (300 V, 13 min)
- Lane order:
- My ULR 1:10, 1:5 new ULR, #4 EP1, y1mL DE form loaded, #3 final form loaded, #3 no glyc 1:10, #1 no glyc 1:10, #2 no glyc 1:10, #4 no glyc 1:10, #5 no glyc 1:10, #4 EP1 b, T7 #4, T7 #5.
- middle of gel melted slightly, loading dye colored bands fainter and distorted.
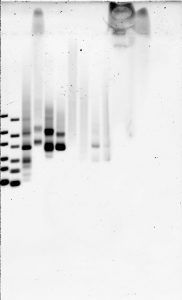
- still only 6 bands! wtf?!
Discussion with Jeff / Planning
Attempt to make columns work better.
- Jeff will make 100 bp dsDNA. I will use to test column recovery.
- should compare this as well to ULR ladder recovery (run before and afer sample on gel)
- probably use small scale columns first. Remember to equilibrate and elute with MiliQ water.
- If this looks good, try with one of the PCR mixes that currently looks good on gel.
Improving denaturing gels
- Need to play with ladders on denaturing gels to get expected bands
- confirm ladders work as expected on Agarose gel first
