9:20 A – 7:00P, 9:30P — 12:20 A
Lab meeting
- visiting post-doc candidate: notes
Probe making
- figure out increased library concentrations for large scale PCR for B/Y/K/G libraries
- set up 5 mL scale PCR for B/Y/K/G using nick-containing primers
Setup
- Blue empPCR adapter product 1:100 + CPPT primer + B-NIP
- Yellow emPCR adapter product 1:100 + CPPT primer + Y-NIP
- Black K1 PCR adapter product 1:100 + CPPT primer + K-NIP
- Ubx emPCR adapter product 1:100 + CPPT primer + U-NIP
CPPT test run
- drop from 70C to 68C annealing temperature, PCR test 60uL sample
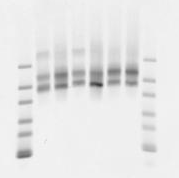
- run out on gel:
testing hand-off PCR
- BXC 0.1 ng/uL
- BXC 0.01 ng/uL
- G orig emPCR library 1:10
- G PCR1 1:100 dilution
- (imaged on gel above)
ethanol precipitation
- only 8 uL glycogen per 5 mL reaction (instead of recommended 80 uL). hopefully it’s not necessary, the DNA yield is large enough as is….
-
- 500 uL NH4OAc + 15 mL cold 100% EtOH. Precipitating at -20C O/N
- old 50 mL scale precipitation marked “Y” and “K” also in freezer — need to check notes.
T7 site addition
- Blue library 1:10 dilution, 1 uL in 500 uL reaction. + Blue NIP primer
- .5 uL T7 primer, .5 uL T7 P1 rc-adapter
- 5 uL T7 P1 rc-adapter
- .5 uL T7 primer, .5 uL T7 P1 adapter
- 5 uL T7 P1 adapter
- .5 uL T7 primer, 2 uL T7 P1 rc-adapter
- .5 uL T7 primer, 2 uL T7 P1 adapter
- small-volume handoff PCRs seem to have extra band. Straight up PCR / higher concentration of long adapter primer seems to give better results.
primer stocks
- Exhausted primers: Y-a,
- Nearly exhausted primers: B-a, K-a,
Probe Butanol extraction
- 7.5 mL butanol to 1.5 mL ddH2O from gel extraction
- vortex well and mix on rocker > 5 min
- 100% of aqueous phase lost into butanol. Oh no!
- added 700 uL ddH2O, recovered 500 uL ddH2O. (should have started with maybe 6 mL butanol and things would have been perfect. Hopefully DNA was recovered as well).
- ethanol precipitating DNA at -2OC O/N
- need to order more glycogen!!
STORM
- Finish O/N STORM ph-wt-rb-647 (still running 90/140 complete)
- ph-FLAG-m-647 + ph-wt-rb-750. 750 staining too weak in intact cell nuclei to see anything
- check on TURNKEY en-Cy3 H3K27me3 (failed, no sginal on STORM2 or TURNKEY)
- STORM en-Cy3 H3K27me3 — Failed, no Cy3 signal. (H3K27me3 looks good).
- attempted to image ph-FLAG-m-750, ph-wt-rb-647. 750 signal still fails. probably a dye problem/deep imaging. Rare sparse switching dots
- recorded >1E6 frames of 647 switching
- Try TCEP buffer next time for 750
- setting up to image PH-FLAG-647, Ph-wt-488 O/N
embryo staining
- wash out secondary from en-Cy3, H3K27me3
Project 2
- Team meeting
- Notes: