9:30a – 7:30p, 9:00p – 11:50p
Meetings
- 10a, lab meeting
- 11:45a, meeting with Ting and Fred
- Need to send letter to team.
Cell in situs
- Wash out primary probes
- STORM E6 (tertiary approach still) — very weak spots visible in some cells
- Spots seem to have some structure in STORM
- STORM F6, very bright dots. some dot background.
- dots pretty bright. Feducials still doing their random vanishing trick on the 60hz data collection.
Probe Making (with Bogdan).
- Black regions RNA clean-up (ran half with beads and half with columns)
- very low RNA yields with both approaches.
- PAGE gel of RNA (lambda control in lane 2, then samples 9 down to 1 (black regions in reverse order of previous log).
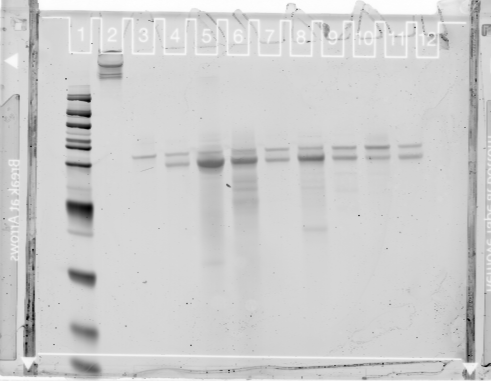
- Troubleshoot tomorrow: I’ll do the RNA cleanup myself and see if the control is indeed low yield.
- maybe PCR contaminates are creating a problem? Had a substantial UV peak and nearly 50% of the volume comes from the PCR cleanup reaction.
small regions
- Check PCRs of small regions
- samples 1-8:
- E02 13 kb green (between R/Y)
- E03 BLUE 13 KB (no genes, good PC/Psc)
- E04 BLUE 10 Kb (flanked by strong PolII)
- E06 Epc (12 kb YELLOW)
- E07 Tou (15 kb YELLOW)
- E09 RED 8 kb intronic tRNA locus, in between blue
- F02 BXC_Y_left (15 kb)
- F08 RED 7 kb region next to black 1, tRNA locus
- samples 9-14:
- F09 BLACK 18 kb region next to RED1
- F12 Taf1_(17kb yellow/green)
- G01 LabRegion_(Lab_Zen2) [83 kb but missing piece of ANT-C]
- G07 AlphaTub84 (5 kb yellow)
- Neg control 1
- Neg control 2
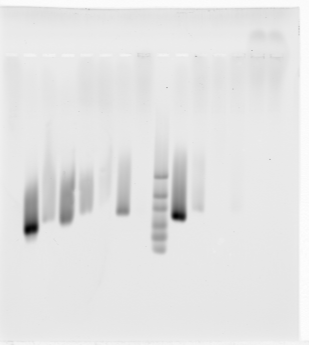
- too much bleaching? caused sample degradation? large volume not as clean?
- not as clean as previous run where some failed but AbdA didn’t. No bubble bands = not promising? Previous lib amplification had bubble bands.
- Repeat PCR, Split into separate 50 uL tubes.
- run G1 and G7 with non-T7 primers
Samples:
- Drop to 50 uL reactions,
- Repeat 1-14 as above (split into 2 50uL reactions).
- E2, G3, G1, G7, Ua
- G1, G2, G7 with non-T7 primers.
- Hao T1, T2, T3 (large library).
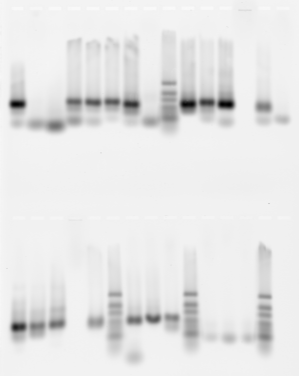
- not all neg controls clean, even on 29 rounds PCR
- ladders not running too clean. Maybe should make a fresh 2% gel and try again tomorrow.
- original library primers amplified well
