9:30 A — 7:00P, 9:00P – 11:55P
- Laser Safety Training 10A-11A
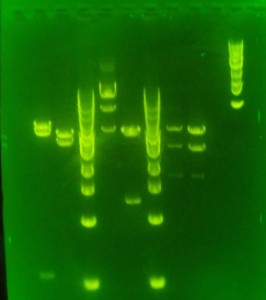
- miniprep samples: toll, y-full, neu3, sxlA, sxl3, sxl1
- Restriction mapping with EcoRI — incubating at 37C at 12:30P.
- Expected fragmentation patterns:
- Neu3: 2x 2.5kb frags + 3 kb Pgem
- Toll: 1 kb and 4 kb frags
- y: 3.3 kb and 1.4 kb frag
- sxl A 5kb
- sxl3: 2.5 and 500bp
- sxl1: 1 kb
- Shown here: toll, Neu3, ladder, sxlA, y, ladder, sxl3 sxl1
- looks like sxl3 colonies got to sxl 1. Not sure why these gels look so smeary. Trying fresh reaction in 2% gel with fresh TEA.
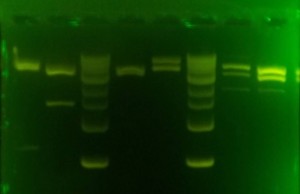
Try alternate 2% gel, longer wells, 100 V, new EcoRI digest 2 hrs.
Straighter lanes. Little improvement, gel ran 1.25 hrs. Lower voltage thinner gel might be better for separating the thick bands.
Other Progress:
- Make small fly plates. Set up new fly cage of ~yw.
- Review setting up DIC on Turnkey, need to collar the field for the appropriate objective with an open light path. — hexagon ring should be in focus and just wider than field of view. Then insert polarizer at top, switch to position 5 the top filter above the condenser, remove eye-piece, adjust top polarizer to minimize light through open eye-piece, replace eyepiece, insert slider below filter wheel from right and slider from left.
- Experiment planning: Test antibodies: H3K27, H3K4, PolII, short fix: hb, bcd
- dissolve in 25 nmol of DNA primer in 0.250 mL of ddH2O to get 100 uM primer soln. Use 1 uL straight for BioXact Taq.
- Download data from Zeiss700 computer.
- Discussion with Josh about imaging.
- 2nd round review to do — started editing.