10:40 A – 8:15 P
- lab meeting canceled due to hurricane Sandy
- contact ML about revisions.
- Got suggestions for graphical abstract, now implemented. Waiting for response on text.
- contact Ting about T7 primers
- Plan to meet with Brian and Fred (Thursday? or next week?)
Polycomb group protein knockdown results
- check scm plate — no emerged larvae yet
- check Psc 1 and Pc 3 plates from yesterday, # hatched larvae ~= # of eggshells.
- check slides from AbdB staining — no AbdB stain evident in Cy3B or 405. Notes from 3/17 suggest this antibody works excellent after strong fix. Maybe antibody gone bad after 6 months at 4C? Should order new antibody and freeze at -20C 50% glycerol. Or maybe fix has gone bad?
- Flip collection plates, 11A.
- Finish fixations from last night (remove heptane, move to -20C sotrage in MeOH).
- check hatching on Scm plate: still no hatching. will check new plate tomorrow.
- clear act5-gal4 into 2 new bottles, moved to 18C for virgin collection.
- evening virgin collection: flipped MTDs, tossed old bottles. collected Act5-GAL4 virgins.
- Flies laying very well. Too late though for fixation, heading home.
S2 cell RNA FISH
- Several wells MeOH all evaporated. Having H2O in incubation chamber doesn’t help the partial pressure of MeOH to prevent evaporation
- added fresh MeOH, incubating at RT.
- move to 50:50 MeOH:EtOH
- move to 50:50 EtOH:PBT
- most cells washed off, morphology not looking great
- PBT + Hoechst: cells look okay, DNA staining evident. a bit sparse.
- 3x PBT wash. -> hybe soln.
- Probes: top row: M2, neur, N3, Epc
- bottom row: cact, N Tkv, blank. Denature at 95C 3 min, add hot to cells.
- Incubating O/N at 55C in hybe oven. Give thorough washing tomorrow.
DNA FISH
- DNA FISH run on coverslips: no cells remain attached after treatment
- Precipitate DNA (forgot to mix well after adding EtOH, partially frozen. Mixing and re-freezing.
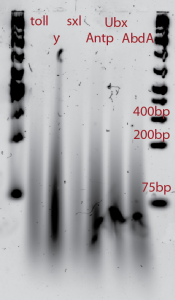
- precipitate, wash, rotoevap dry (Ubx and Sxl resuspended, pellet somewhat liquid, dissolves instantaneously. May be some alcohol/H2O still not evaporated. Put other samples back in rotevap).
- Secondary evaporation: probes change to lighter color, clearly all EtOH is gone now. Previous Ubx Sxl probes may not be so good. Should plate some cells to try these probes on?
- New probe synthesis for all 6 probes, 2 hr reaction. Dilute enzyme mix 3:2 with PolI mix (previous probe size mostly 0-200, desired probe length 200-400).
- short weak probes:
Revaluating Cell Culture approach
- Adherent cell lines might be better?
- tried spinning down cells, resuspend and move to small culture chamber with new media.
- took cells from healthy 10-24 culture chamber (good morphology, high fraction attached, good density), and plated in new small culture chamber.
Snail
- clear E(spl)/sim[D] cages for renewed virgin collection
- cross current virgin E(spl)/sim[D] to male TM2/TM6 (multi-fly crosses. next gen is single fly crosses to TM2/TM6, will need virgins from that line to be able to use males so let’s keep expanding the double balanced 3s. After this next generation, the line that able to kick the balancer out has siblings who are double mutants).
- collect evening E(spl)/sim[D]
