9:15 A — 7:15 P, 8:15P – 1:15 A
Cell labeling
- hot washes for probe first slide (2x at 60C)
- 1x at RT
- remove and save primary antibodies at RT for primary first slide.
- PBT rinses (3x soln change, 3rd wash incubating over lab meeting).
- antibody first: — stains in all antibody channels in rb-488 look good. 405-750 okay too?
- Psc brightest of PcG proteins (didn’t do Pc), also most puncate. PCNA weak, maybe puncta in some cells, shoud do another survey on the confocal. H3K9me2, some cells have pronounced puncta, we’ll see how this colocalizes with cent-DNA.
Graham lab meeting. Notes
Fly Work and DNA screening
- new crosses for recombination. 100 single fly crosses of Espl,sim possible recombinants x TM2/TM6 double balanced flies
- PCR screen deletions.
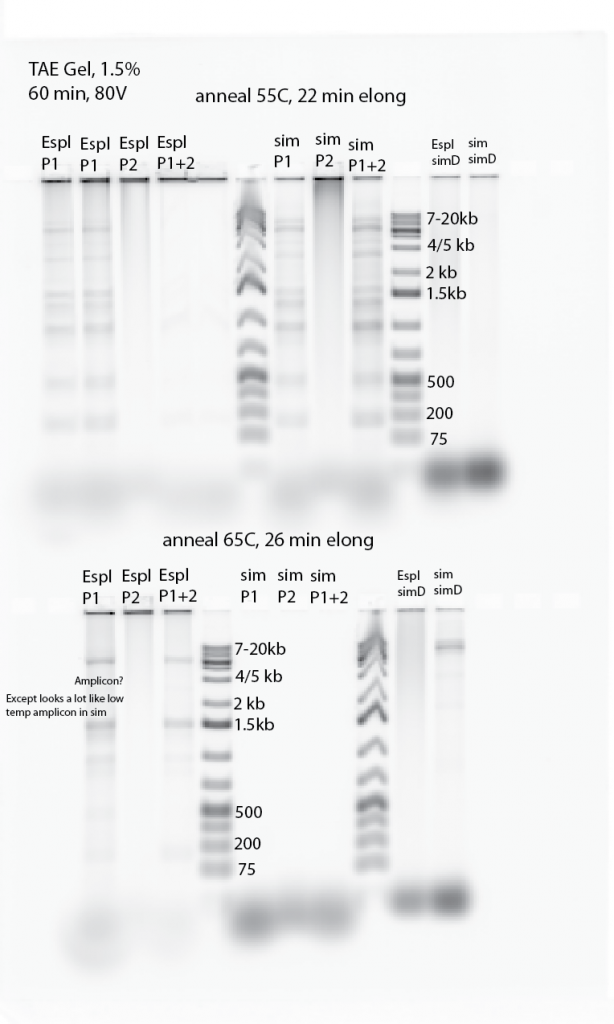
- Designing new primers using Primer3 tool for Espl and sim.
- Espl has ~400 kb deletion with uncertain boundaries. Should just be able to tile a bunch of forward primers broadly across the predicted left arm break and bunch of reverse primers broadly across the right arm break. Combine all primers in 1 tube under high spec reaction conditions and should be good to go.
- Primer3 chokes on 30kb output 20 primer request. Try 12kb output 15. –> Needs to be 5-6kb max it seems.
- Primers for sim[D]. primer3 output
- order primers.
Probe Making
- Set up digests
- running digests in PCR
- Freeze O/N. (precipitate tomorrow?)
- Write to Bryan about finding new time for PAGE. — wed afternoon.
STORM2
- background / thresholds all need to be substantially higher
- 647 good switching.
- H3K9me2-750 poor switching. Not much signal. Few punctal spots. H3K9me2-488 pretty vague focus.
imaging cells:
