Amplifying G1-8 using T7 primers
for each sublib
- 18.5 ddH2O
- 5 uL 5uM common
- 1 uL library (cut to .5, at 1uL I can only do each lib once)
- 25 uL Phusion master
master mix (9x)
- 166.5 uL ddH2O
- 45 uL common
- 5 uL lib
- 225 uL Phusion
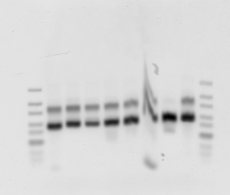
G1 to G8 loaded left to right.
Original PCR (non-T7 primers).
 <a
<a
Gel 1-8. (Forgot to run ladder):
href=”http://alistairboettiger.info/wordpress/wp-content/uploads/2013/08/PCR_lib2.png”>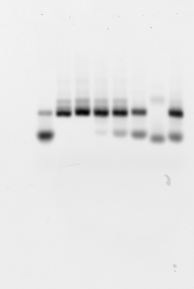
Rerun with ladders after column purification. Confirm that 40mer primers still vanish. G1 and G7 no good (maybe G7 not well loaded? interesting that there is no smear in these ones. Or not well mixed original library?
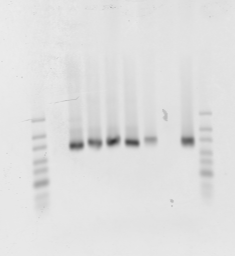
What happened to the bubble-band ? (or the non-bubble-band?, based on fragment size.)
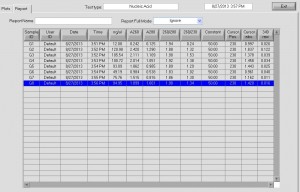
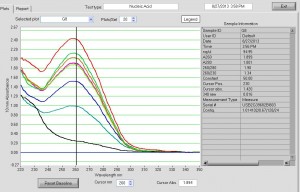
Nanodrop