Library negative controls
- Get 10% bleach for spray bottle.
- Bleach all pipettes. Get all new tips
- 25 uL Phusion reactions
- 10 uM primer dilutions
- Fresh library 1:100 dilution 1 uL + 1.25 uL 10uM common
- Fresh library 1:100 dilution 1 uL + 1.25 uL 10uM common + 1.25 uL 10 uM AbdA-T7
- Fresh library 1:100 dilution 1 uL + 1.25 uL 10uM common + 1.25 uL 10 uM AbdA-F
- Fresh library 1:100 dilution 1 uL + 1.25 uL 10uM common + 1.25 uL 10 uM E6 (non-T7)
- Fresh library 1:100 dilution 1 uL + 1.25 uL 10uM common + 1.25 uL 10 uM E6-T7
- Old library with common.
- Old library with common + 1.25 uL 10 uM AbdA-T7
- Old library with common + 1.25 uL 10 uM AbdA-F
sample 1-8 from right to left
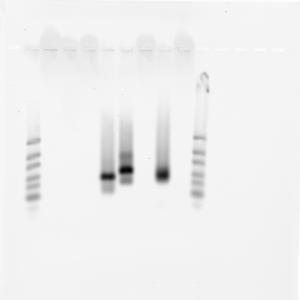
Results
- AbdA-T7 + old library with common is contaminated.
- AbdA-T7 with new library is clean
- E6 +/- T7 are both clean. E6 non-T7 has no bubble band.
Setup with Bogdan
- Overview chromatin project
PCR of black domains
- 10 kb black domain: D09, next to Red3
- 10 kb black domain: F09, next to RED1
- 50 kb black domain: G08, between GRN and ANTC
- 50 kb black domain: E05, upstream of E(Pc)
- 100 kb black domain: F01, BXC_K_left, 125kb,
- 250 kb black domain: F07, BXC_K_right, 240kb,
- 250 kb black domain: F11 ANTC-L 180kb
- 500 kb black domain: F10, 478kb region, on 3R.
- 500 kb black domain: G10, 425 kb on X
- AbdA-T7 negative control
mRNA project
- designing deeper primer sets for mRNA project
- testing length screening,21-25mer, TM 70-80: 8471, 10893, 12662, 14040, 16329
- remove 2 out of 3 terminal GCs to 1 out of 3 terminal GCs.
- running BLAST to genomes independently
cell culture
- Fresh media for both vials of Kc167 cells
- newer vial is adhering much better, should passage these soon.

