8:30a – 12:20a
Morning tasks
- give Jeff deep-seq prep’d sample 4 (F3) to run as qPCR standard
- All analysis tasks on Cajal and all split-dax running from Monet completed.
- Restart Monet to install new 4Tb HDs: ProBox5 & 6. Move ProRAID to it’s own USB3.0 input.
- Power suddenly dropped to 50 mW during movie 15 of E10rna on STORM4. Reset back to 500 mW during movie 25 this morning (only 2 more movies left at the time). Could have been worse I suppose. No idea why the 642 power resets.
STORM Analysis
- Computing more chromatic warps to be able to align bead fields to do image subtraction
- Processed Ydc, Bdc and Gdc in ChromatinCropper.
Coding
- added safety checks to ReadMasterMoleculeList and ReadDax
- added multicolor conventionals to ChromatinCropper (with chromatic correction).
- added bead subtraction to ChromatinCropper.
Deep sequencing prep
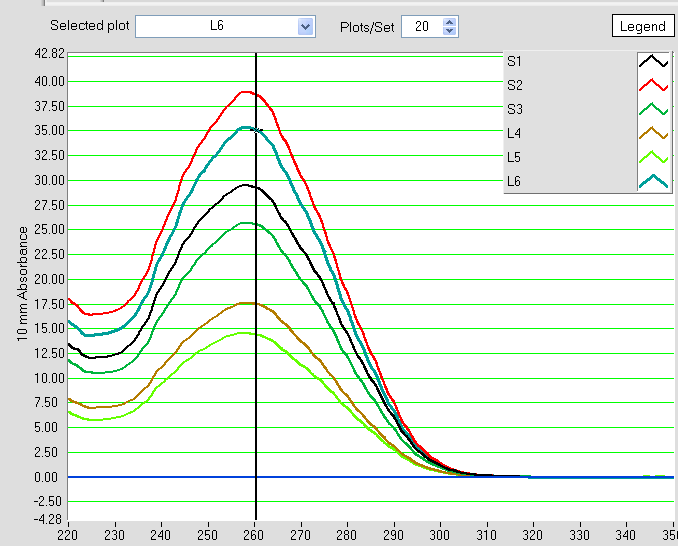
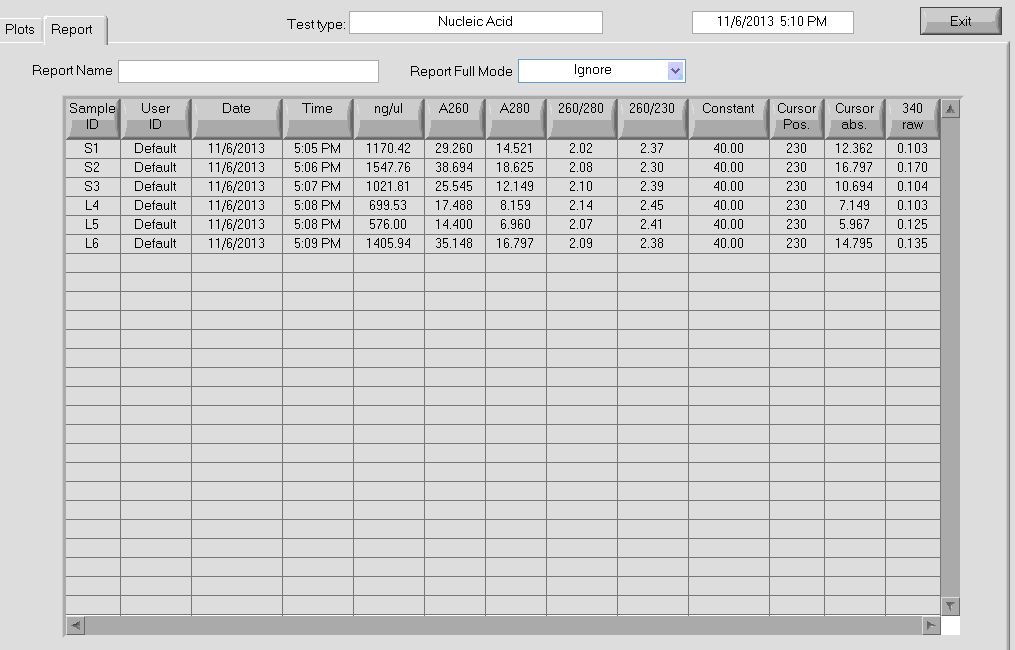
- corrected concentration calculations (missing factor of 2 from doing 10 uL scale with same total amount of standard).
- concentrations for my library (average across dilutions and replicates)
id# conc (pM) CV
1 60 0.25 <— This sample we will exclude
2 6e+04 0.065
3 2.9e+04 0.051
4 1.9e+04 0.18 <— this is the sample Jeff tested (estimated conc 1.7e+04 pM))
5 6.1e+04 0.035
6 3.9e+04 0.052
7 2.6e+04 0.063
8 8.3e+04 0.048
9 3.5e+04 0.062
10 4.6e+04 0.1 <— These are my complete libraries
11 2.6e+04 0.077 <— These are my complete libraries
12 3.1e+04 0.049 <— These are my complete libraries
Probe Making
Estimating primer amounts for each reaction.
New short probes
| Id | name | DNA template (ng/uL) | RNA yield (ug) (2x amount measured in 10uL) |
|---|---|---|---|
| 1. | D07 9 kb Green | 75 | 80 |
| 2. | E02 13 kb green | 150 | 120 |
| 3. | E03 BLUE 13 kb | 110 | 80 |
| 4. | E04 BLUE 10 kb | 70 | expected: 80 |
| 5. | E05 BLACK/blue 54 kb | 80 | expected: 80 |
| 6. | F08 RED 7 kb | 80 | expected: 80 |
| 7. | F12 Taf1 (17kb yellow/green) | 130 | expected: 120 |
| 8. | G07 AlphaTub84 (5 kb yellow) | 50 | expected: 40 |
moderate size regions
| Id | name | DNA template (ng/uL) | RNA yield (ug) (2x amount measured in 10uL) |
|---|---|---|---|
| 1. | D08 GREEN 50kb | 30 | Estimate 120 |
| 2. | D10 RED 50kb | 30 | Estimate 120 |
| 3. | E03 BLUE 13 KB | 30 | Estimate 120 |
| 4. | E11 50 kb GREEN | 13 | 40 |
| 5. | F03 Ubx_through_bxd | 8 | 40 |
| 6. | G04 Antp | 22 | 120 |
RT reactions
- 500 ng/uL in 40 uL -> 20 ug RNA. -> 300 pmol of primer
- 1000 ng/uL in 40 uL -> 40 ug RNA -> 600 pmol of primer
- 1500 ng/uL in 40 uL -> 60 ug RNA -> 900 pmol of primer
- short probes: 8 gets 300 pmol of primer. 1,3,4,5,6 all get 600 pmol of primer. 2 and 7 get 900 pmol of primer.
- long probes: 1,2,3,6 get 900 pmol of primer. 4 and 5 get 300 pmol
- 10 uL of RNA + 3 – 9 uL of primer + 6 uL of 10mM dNTPs + 7 – 1 uL of ddH2O (26 uL)
- 16x buffer master mix (40 uL rxn volume):
- 2 uL of Maxima + 10 uL RNase inhibitor + 128 uL of 5x buffer + 22 ddH2O
To order
- More Maxima
- more 40bp common A405