9:00 am – 7:30 pm, 9:00 pm – 11:30 pm
Thesis defense
- finalizing hat for Hao 9am-10am
- Hao’s Thesis defense 10am-11:15 am
- more finalizing hat 11:15-11:45
Discussions 11:45-1:00pm
- discussion of AO with HB and BB
- discussion of BB’s sequential stain data — look’s awesome!
Presentation Prep
- working on slides for CSHL meeting
- working on slides for Ph-polymerization project summary for tomorrow lab meeting
MERFISH
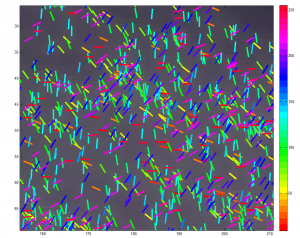
Developing New Pipeline for Multicolor data 7:00pm – 11:20 pm
- attempting pre-warp
- using brightfield bead movies and CorrAlign
- adjusted
CorrAlign to dilate images before computing correlation
- this is computationally expensive. 5x is quite slow. 3x is okay.
- this does provide sub-pixel improvement
TranslateImage however is pixel based, doesn’t provide sub-pixel translation.
- could make sub-pixel version — just dilate, then translate.
Questions for team
- why 3 color 8 hybes for L7 (only 16 bits not 24…)
- there are clearly focus lock issues still…
- why is 10 uM scale of primer $400!! (it would be cheaper to order 10x 1uM scale, ~$300)
Ph Project
- reply to NJ about abstract
- to do tomorrow: rewrite model discussion section
- re-arrange the rest of the Fig 5 panels