November 2025 M T W T F S S « Aug 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Categories
- AP patterning (13)
- Blog (1)
- Chromatin (88)
- Conference Notes (72)
- Fly Work (54)
- General STORM (25)
- Genomics (134)
- Journal Club (22)
- Lab Meeting (66)
- Microscopy (79)
- Notes (1)
- probe and plasmid building (58)
- Project Meeting (3)
- Protocols (13)
- Research Planning (74)
- Seminars (21)
- Shadow Enhancers (59)
- snail patterning (40)
- Software Development (5)
- Summaries (1,412)
- Teaching (9)
- Transcription Modeling (40)
- Uncategorized (10)
- Web development (19)
Links
Tags
analysis cell culture cell labeling chromatin cloning coding communication confocal data analysis embryo collection embryo labeling figures fly work genomics hb image analysis image processing images in situs Library2 literature making antibodies matlab-storm meetings modeling MP12 mRNA counting Ph planning presentation probe making project 2 project2 result results sectioning section staining shadow enhancers sna snail staining STORM STORM analysis troubleshooting writing-
GitHub Projects
Wednesday 08/06/14
9:15 am – 9:15 pm
Ph project
- discussion clarifying “cluster” definition — yes this goes all the way down to “clusters” which probably contain only a single molecule.
- analyzing PH clusters lacking PC
- this is not as robust with the 750 data as with the 647 data
- need some adjustments for background and density
Project 2
- team meeting
- data analysis — see post.
Chromatin imaging
- need new z-calibration movie
- modify CC to use bk images for mask and original scan images for conv. Ideally have toggle to use either set in either place.
- currently 2 channel nature of the data is not detected if we use single channel conventional images.
- fixing some issues with chromatic alignment — turns out I entered the channels in the wrong order in STORMrender, and then got myself confused. CC and STORMrender agree perfectly, CC for the 750 data takes the guesswork out.
Chromatin data analysis
- analyzing F06 F07 data
- 750 background pretty high. Not certain the z-focus difference is perfectly corrected
- new 750 z-calibration seems to be needed.
- despite skipping lots of spots for background issues or low localization number, I can still get 50 cells in this data set.
Posted in Summaries
Comments Off on Wednesday 08/06/14
Tuesday 08/05/14
9:30 am – 10:00 pm
STORM
- L2 F02 F03 — F03-p3-750 not very bright given size
- F02 stain looks like it could be better — a bit of background here too
- imaging L2 F02 F03 anyway, shorter exposure
- 9 pm set up imaging of L3 E08-p2-6 E09-p3-7
- not as bright as expected for these loci. Remade Glox buffer (6 days old now), spots brighter
Probe Making
- ChrLib4 probe synthesis A01-B12
IVT reactions
- 30 uL total :
- 10 uL buffer nucleotide mix
- 10 uL DNA template
- 1 uL RNAsin Plus
- 1 uL T7
- 8 uL ddH20 nuclease free
25 x master mix
- 250 uL buffer nucleotide mix
- 25 uL RNAsin Plus
- 25 uL T7
- 200 uL ddH20 nuclease free
RT
- 3 uL primer
- 2 uL dNTP
- 14 uL RNA
- 6 uL RT buffer
- 1 uL RNasin
- .5 uL Maxima
- 4 uL ddH2O
25x RT P2
- 75 uL primer P2
- 50 uL dNTP
- 150 uL RT buffer
- 25 uL RNasin
- 12 uL Maxima
- 100 uL ddH2O
6x RT P3
- 18 uL primer P3
- 12 uL dNTP
- 36 uL RT buffer
- 6 uL RNasin
- 3 uL Maxima
- 24 uL ddH2o
- tubes 1,3,8,17,19
cleanup
- froze samples, try plate-column cleanup tomorrow.
PH polymerization
- working on manuscript revisions / additional data analysis.
- quantify total overlap by fraction of localizations of one channel within localization precision (~25 nm) of other channel. I had done this for PH-WT and not PH-ML before. Quick estimate drawn from last cell of PH-ML data (20% PH-ML with PC and 30% PC with PH-ML). Should go back and compute all cells and average.
- quantifying distribution of non-co-clustered populations — this we basically had already, just plot the
~coclusteredmlists and run cluster size stats on those. - quantifying correlation of cluster sizes. — finished off analysis started yesterday. Approach uses an OR mask and then overlap to call clusters, so all localizations from two clusters that share a portion of pixels are counted — this reduces artificat from partial misalignment on the 20-50 nm scale due to residual uncorrected drift and residual uncorrected chromatic aberration.
Other Notes
- new GSA membership
Posted in Summaries
Comments Off on Tuesday 08/05/14
Monday 08/04/14
7:30 am – 5:15 pm, 10:15 pm – 12:20 am
Chromatin STORM
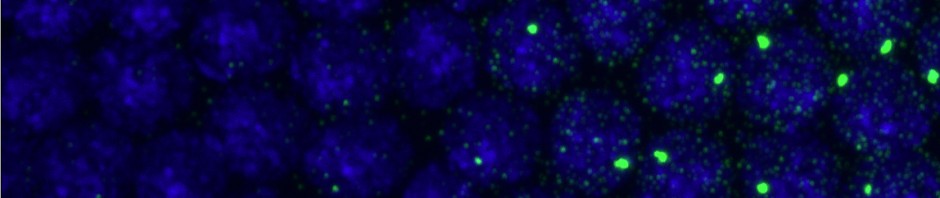
- setting up imaging of L2 F01-A750 F02-A647
- a bit dim but clearly detectable
Project 2
- working on data analysis with Steven’s new data.
- see protected post.
(volleyball – 11:00am-12:30pm)
PH project
Manuscript
- new manuscript draft from Bob/Nicole/Ajaz
- some additional modifications to the figures coming soon.
Data analysis
- look at size distribution of non-overlapping clusters.
- analyzing size correlation in PH-PC clusters
Posted in Summaries
Comments Off on Monday 08/04/14
Sunday 08/03/14
10:15 am – 8:00 pm
Probe making
- out of RNasin, out of Maxima, not able to proceed with probe making.
- really need to have back ups of these.
Coding
- started to write SteveMosaicViewer GUI
- drag and drop of a mosaic file will create run
ConvertStv2Matif needed and create astvfileglobal that can be loaded - rest of render still needs work
- fixing issues with Mosaic tiling x,y definitions, transposes etc.
- odd issue with tiling: overlapping info:
STORM
- imaging E06 E07 two color
- same buffer as yesterday: 1.5 uL BME, 2.5 uL MEA, 5 uL COT, 10 uL GLOX, 500 uL im buffer with glucose.
- spots look pretty good.
- mosaic scanned using 647 channel.
- now recording channel in filename
New Stains
- (.5 uL of secondary and 2 uL of primary each)
- L2 F01-P3-A750 + F02-P2-A647
- L2 F03-P3-A750 + F02-P2-A647
- L2 F05 + F06
- L3 E08 + E09
Saturday 08/02/14
10:00 am – 9:00 pm
STORM
L2 F06F07
- finish hot-washes
- add beads, make STORM buffer
- Imaging F06-p3-7 F07-p2-6 double color
- staining in both channels bright and clear
- colocalization looks decent
New buffer for multicolor STORM
- 2.5 uL MEA
- 1.5 uL BME
- 5 uL COT
- 10 uL GLOX
Writing
- writing up formal comments on manuscript for colleagues
- # need to write abstract of DR retreat!
New Probe making
regions
- internal black domains: A01-A10 + E01-E10
- some BXC small pieces: A11-B12 + E11-F12
- 24 new probe regions in all (plus let’s do some in 2 color).
per well
- 25 uL 2x Phusion master hot start
- 2.5 uL Eva Green
- 5 uL library
- 12.5 uL ddH2O
- 2.5 uL Fwd Primer
- 2.5 uL Rev Primer
27 x master mix
- 675 uL Phusion hot start master mix
- 67.5 uL Eva Green
- 135 uL library
- 337.5 uL ddH2O
Cleanup
- ran cleanup using Zymo 96 well plate.
- much easier than individual spin columns
Posted in Summaries
Comments Off on Saturday 08/02/14
Friday 08/01/14
7:30 am – 10:00 pm
(9:30 am – 11:30 am volleyball)
RT reactions
Probes in pipeline
- L2 F01 – F07
- L3 E06 – E09
- making with both P2 and P3
Per RT
- 15 uL RNA
- 4 uL primer
- 6 uL RT buffer
- 2 uL dNTPs
- .5 uL RNAsin
- 2 uL ddH2O
- 0.5 uL Maxima
- Total volume = 30 uL reaction
12 reactions master
- 48 uL primer
- 72 uL RT buffer
- 24 dNTPs
- 6 uL RNasin
- 6 uL Maxima
- 12 uL ddH2O
- now OUT of Maxima
- now OUT of RNasin
digestion and cleanup
- added 2x volume of EDTA + NaOH (equal volume would have been sufficient)
- volume for cleanup now greater than 1 column
- accidently starting adding on P2 probes E6 through E9 in backwards order (should have kept this in ordinal order at the T7 stage).
- Lib
Test gels
- all my precast gels have been used
- started casting new gels
- acrylamide won’t polymerize today
- added more TMED and APS, still no polymerization
Cell staining
- new stains:
- F06-p2-A647 + F07-p3-A750
- F07-p2-A647 + F06-p3-A50
Posted in Summaries
Comments Off on Friday 08/01/14