9:45 A – 8:30 P
- O/N hybridization — should do in PCR block. Water evaporates from 37C block, not good contact with PCR tube. Refilled water, allow another hour of hybrdization before washes.
- Sequencing results: A1 = Taf1 antisense (sequence with m13F = T7 side) use T7 for antisense probe
- A2 = Taf1 sense -> use SP6 for antisense probe
- U1 = alphaTub sense -> use SP6 for antisense probe
- U2 = alphaTub48b antisense (sequence with m13F). has 500 bp homology with aphaTub84D (similar genomic location)
- setup PCR to linearize Taf and Tub for probe making:
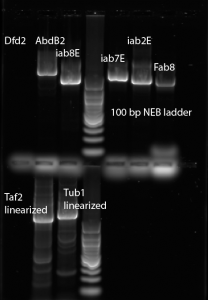
- Repeat PCR for BX-C probes: Fab8, iab2E, iab7E, iab8E, AbdB2, Dfd1, try 60 annealing instead of 72C. max probe length is 2kb, try 3 min elongation.
- setup probe making reactions for en and hox loci. KE didn’t label gel extracts, just ID numbers. ID constructs by size:

- Running test gel of BX-C cloning round 2:
Dfd1, AbdB2, iab8E, iab7E, iab2E, Fab8 (respectively) - Running test gel of PCR linearization of Taf and Tub templates (respectively)
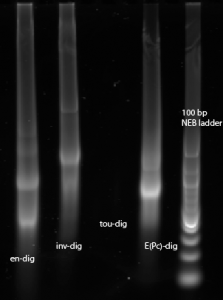
- Running test gel of in vitro transcription reactions of en, inv, tou and Epc (respectively)
- Make 50 mL STOP soln. Use RNAse free H2O
- Make 50 mL 2x Carbonate buffer. Use RNAse free H2O
- Blocking hybridized embryo
- incubate with ch a-GFP, m a-Dmo, rb a-H3K27