10:30 A – 7:30P, 8:30P – 11:45P
Lab meeting prep
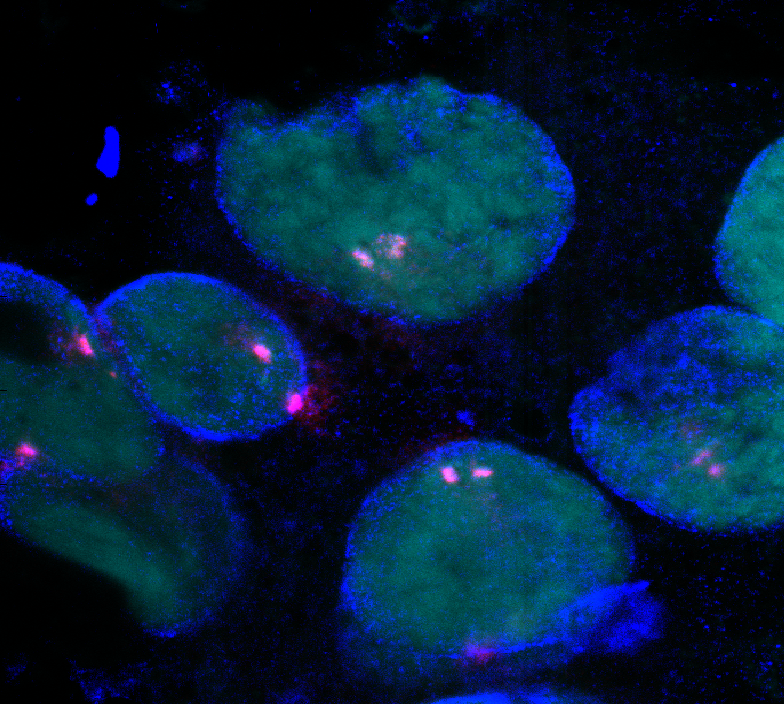
- collecting dual labeled images to show attempts with flanking probes.
PCR
- Test run gels from Espl screening. Gel 1 from BioXAct (3A and 4A, see legend in yesterday’s post), numerous weak off target bands, all bands match in both control and Espl lanes.
- Gel 2 10 min LongAmp PCR, 10kb band in what I thought was simD missing in EsplD, maybe the PCR tubes got reveresed, should have labeled them these ones lack polarity. Try to extract, PCR amplify and sequence.
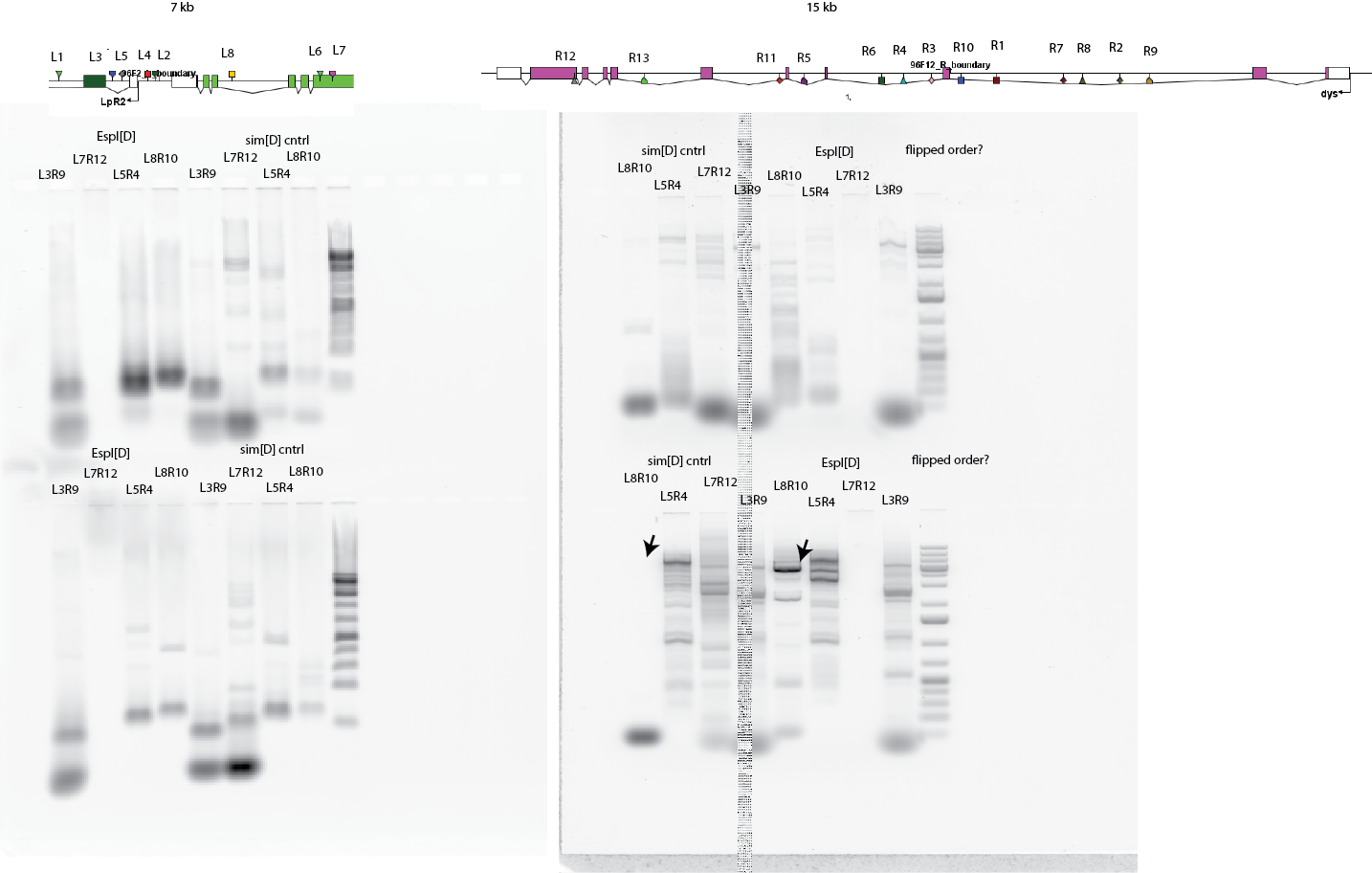
- Extracting heavy band from marked lane. Will try to reamplify and sequence.
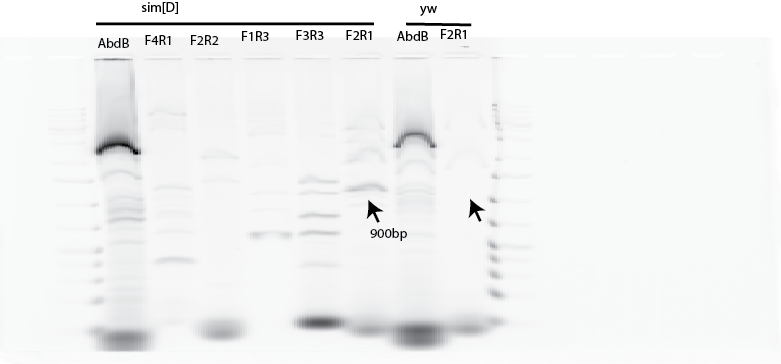
- Potential band across sim deletion (TAE gel ran better + imaged ladder, but I didn’t remember to convert the file format. Not sure why the Borax gel in this case is running sad faced. But the fragment unique to sim[D] and is of the expected size for the F2 R1 primer pair:
Probe Making
- Precipitate dissolved gel – 80C 1 hr
- forgot glycogen. rewarm, add glycogen. Doesn’t appear to help after adding Ethanol, glycogen percipitates immediately in cold EtOH.
- second round of incubation of ddH2O in ground up gel/DNA extract, percipitated in proper order (also 4 tubes with 50uL ammonium acetate, 3 uL glycogen, and 1.3 mL cold EtOH).
- reprecipitating at -20C 1 hr.
- Resuspended in ~120 uL ddH2O, ready to repeat denaturing gel. Remember to denature fragment this time at 95C immideately prior to loading.
Embryo Fish on UltraCryo Sections
- Take ‘before’ pictures of embryos in DAPI. Most sections nice and flat. Good number of sections on slide. (mostly young embryos).
- Incubate in SSCT, move up to prehybe, prehybe at 60C in hybe oven >30 min
- denature in hybe oven at 90-95C, 10 min with ~100 uL probe (1:500 reused aliqout in embryo DFISH buffer)
- stain O/N at 37C
Embryo FISH
- move from PBS-Tr to prehybe,
- prehybe at 80C, 14 min
- Add probe (1:200 in DFISH buffer), react O/N.
Sectioning prep
- polylysine coate 24×30 mm coverglass in coplin Jar using 0.1-0.2% polyLysine-D. (unfortunately this ancient lab bottle of polyLysine powder says store at -20C and has been kept at room temp for years. Maybe someday the fresh poly-Lysine solution that I ordered will come in and my hard cut sections will not drift away).
- Air dry slides. some percipitates of lysine but not as bad as the salt percipitates from the PBS on the last set of slides coated with this solution.
Processing polytene cell images
Confocal
- Imaging SCM mutants
- Imaging Dfd-tub for distance measurements