9:30 A – 8:00 P, 9:00 P – 11:55 P
Meetings
Gels
- At least 3 ways to screw up gels
- APS really needs to fresh, (try 140 uL instead of 38 uL)?
- mix up BME and TMED
- nicely cast gels don’t refirgerate — Urea percepitates and creates air bubbles.
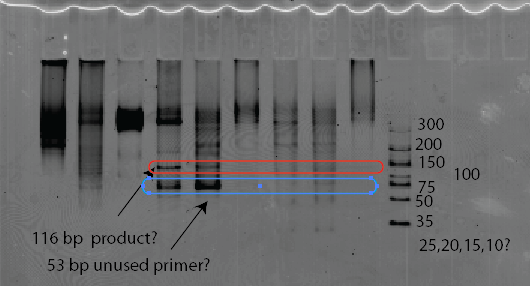
* (gel lanes as numbered below, 1-10 left to right).
Nanodrop
- y nicked 2x Ya-primer [358 ng/ul]
- b nicked 1x Ba-primer + 1 x CPPT [363]
- ubx nicked 1x UBx-adapter-primer + 1x CPPT [360]
- y PPT: 5 uL YA + 20 uL PPT [356]
- y PPT control: 5 uL YA + 20 uL CPPT [354]
- y assym: 10 uL YA + 10 uL CPPT (No YR) [355]
- b PPT : 5 uL BA + 20 uL PPT [360]
- b PPT control: 5 uL BA + 20 uL CPPT [359]
- b assym: 10 uL BA + 10 uL PPT (no BR) [346]
- 10 mM dNTPs 5,330 ng/uL
Testing cleanup column
- (#4) 150 uL of y PPT PCR prod + 750 uL binding buffer
- (#5) 150 uL y CPPT PCR prod + 750 uL binding buffer
- Doesn’t look like any DNA detectable on denaturing gel.
New project with Xiaowei
- setting up coding with Steven
- sent project schema outline to team
