November 2025 M T W T F S S « Aug 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Categories
- AP patterning (13)
- Blog (1)
- Chromatin (88)
- Conference Notes (72)
- Fly Work (54)
- General STORM (25)
- Genomics (134)
- Journal Club (22)
- Lab Meeting (66)
- Microscopy (79)
- Notes (1)
- probe and plasmid building (58)
- Project Meeting (3)
- Protocols (13)
- Research Planning (74)
- Seminars (21)
- Shadow Enhancers (59)
- snail patterning (40)
- Software Development (5)
- Summaries (1,412)
- Teaching (9)
- Transcription Modeling (40)
- Uncategorized (10)
- Web development (19)
Links
Tags
analysis cell culture cell labeling chromatin cloning coding communication confocal data analysis embryo collection embryo labeling figures fly work genomics hb image analysis image processing images in situs Library2 literature making antibodies matlab-storm meetings modeling MP12 mRNA counting Ph planning presentation probe making project 2 project2 result results sectioning section staining shadow enhancers sna snail staining STORM STORM analysis troubleshooting writing-
GitHub Projects
Tuesday 07/15/14
10:45 am – 9:55 pm
Teaching
- training Bogdan on Cryo
- cutting mouse brain. Target size 1um
- initial cutting temperature = -55 C
- sections look reasonable with DAPI.
- collecting sections for imaging
- teaching proper clean up procedure
- New supplies to get
- backup small perfect loop.
- brushes
Chromatin
Sequential staining
- S3toe5 failed quality control, won’t ship until tomorrow
- S1toe5 shipped last night, should be here to test with
- can I do S3 after S1? This actually has 15 bp of homology because of the shared 10 bp.
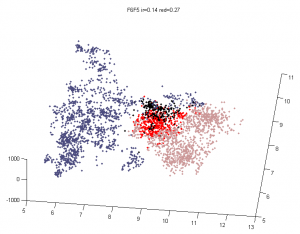
- imaging BXC-S1. At least these look like some good BX-C images.
Internal scaling
- selecting and cropping sample images for figures.
Posted in Summaries
Comments Off on Tuesday 07/15/14
Monday 07/14/14
10:00 am – 10:55 pm
STORM
- imaging D11
Chromatin Project
- New stains
- L3E06
- L3E07
- L3E07toE09
- L2 F03toF04
- L2 G02toG04
- New stains F3toF5 P1 + F6-P3, 2 large coverslips
Chromatin overlap
data rendering
Models to compare
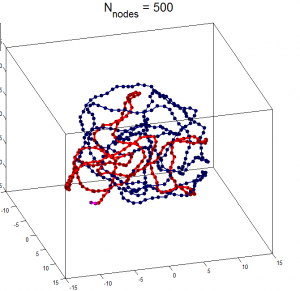
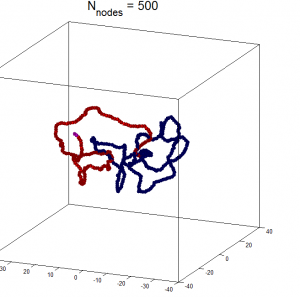
- Components of a random walk polymer
- Components of a random walk polymer with interactions
- Components of an intact, spatially constrained domain, equilibrium globule folded.
- internal components of a spatially constrained domain
Different strengths of volumetric constraint
Issues with current simulations
- unable to get fractal packing from volumetric constraint. Instead get knots
- energy minization starting system in knotted states?
- rigidly exclude branch crossing?
- need to relax system progressively?
Friday 07/11/14
10:00 am – 3:30 pm
Project 2 data analysis
- see notes
Posted in Summaries
Comments Off on Friday 07/11/14
Thursday 07/10/14
10:00 am – 7:30 pm, 11:20 pm – 12:10 am
Post-doc candidate visit
- see notes
Chromatin project figure making
internal domains
- need examples for figures
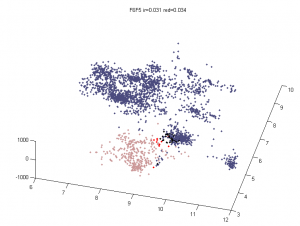
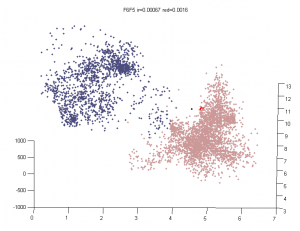
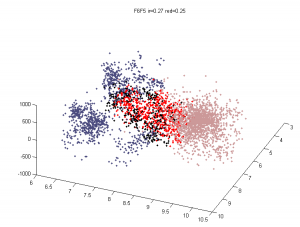
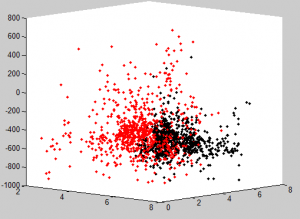
Multi-color region analysis
- Black:Yellow
- F01 :: F02
- F07 :: F06
- F11 :: F12
- G08 :: G06
- Blue :: Black
- G08 :: G09
- Blue :: Yellow
- F03 :: F02
- F05 :: F06
- G01 :: F12
- G05 :: G06
- Blue :: Blue
- G01 :: G02
- G02 :: G03
- F03 :: F04
- F04 :: F05
- Yellow :: Yellow
- L3E06 :: L3E07
- L3E07 :: L3E08 (?)
- Black :: Black
- L3D11 :: L3D12
Analysis methods
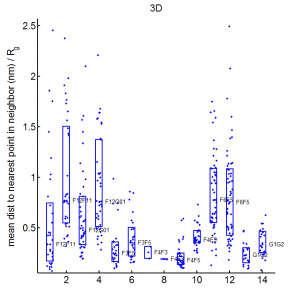
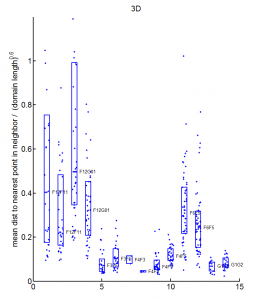
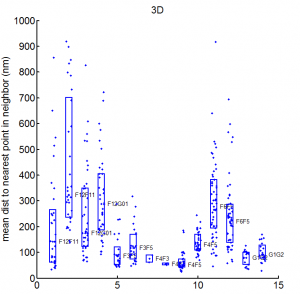
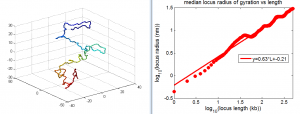
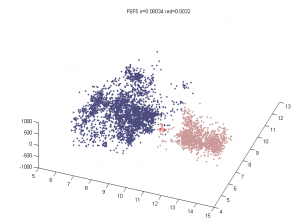
- want an Rg analog for overlap — we don’t want differences in the fringes of scattered particles to make a difference in the ‘volume’ fraction overlap.
- to be Rg analog, metric should treat localizations as density
- We could also make this independent of binning — which was an annoying free parameter last time.
- Proposal:
- fraction of localizations in one channel with at least N localization within distance D (relative to total localizations making up domain).
New simulations
- Free polymer with spherical confinement to a default density of 0.3 particles per cubic nanometer (if bond size is 1 nm).
Protected: postdoc interview 07/10/14
Posted in Lab Meeting
Comments Off on Protected: postdoc interview 07/10/14
Wednesday 07/09/14
9:30 am – 11:30 pm
To do (Oligo Secondaries)
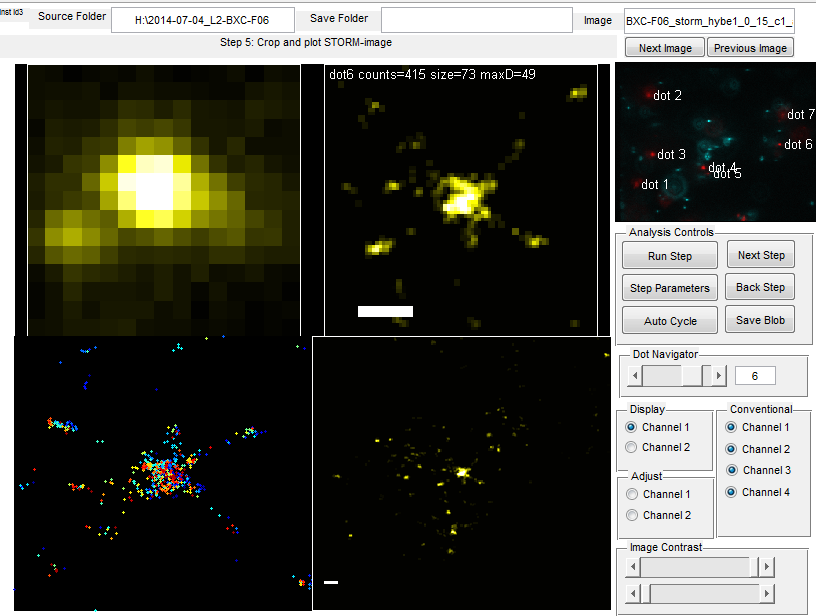
- review new fig 2 layout for Brian
- send additional images of spots for Fig 14 for Brian
Sequential staining
- validated that toe-hold probes do work, competitive probes do not work, (for displacing oligos).
- F06 high concentration cocktail kind of works, not nearly as bright or as many localizations as I expect though for this fairly large region
- (30K frames is all I got).
- Maybe F06 P3 probes work better, I seem to have a lot more batches of these.
- Imaging F06 regions. (just a handful of short movies, so that I can test the washout and BXC staining today too).
- keeping 405 laser below 10% max power.
- ah, second hybes really not working (at least after treatment with competitive probe. Though I have trouble believing that is the problem.
- Should try again with 1st hybe on scope.
- should try again with just bleach no toe-hold probes.
Datasets
- 14-07-04 data:
- rapidly gets too sparse sampling: (buffer rundown?)
- consistent with superposition of F06 and BXC (both P3 probes)
- 14-07-02 data
- much of this data is also rather sparsely labeled, especially given the contrast at brightness of the spots
- this data also shows clear signs of buffer run down, images 7 on notably fewer localizations
- chamber will need to pause and flow fresh buffer more frequently for the long term imaging this way.
Chromatin To do:
- compute total number of loci imaged in final data set
- compute total number
- compute number of genes covered
Internal domain analysis
Blue data
- With region size having such a weak effect for large domains on total domain area (for internal regions), we really need to define the medians very well in order to make good fits. This will require substantially more data than we needed for the complete domain scalings
- new regions to do:
- F03toF04
- F04toF05
- G01toG03
- G01toG04
- should also look at small pieces of BX-C using new library
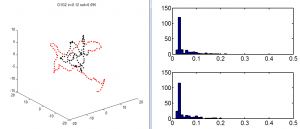
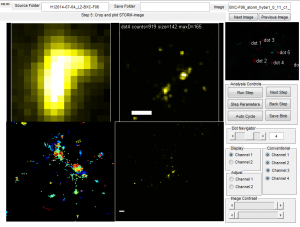
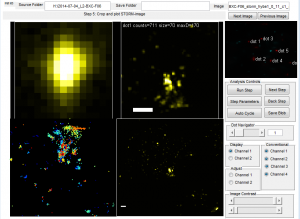
Deeper analysis of Y-internal domain data
- E06 data pretty noisy
- E07 data rather sparse (just 3 images on each of 2 different systems)
- E06E07 data is actually quite good, but needed to do better on censoring the out of focus images. — elongated x-y blurry blobs no good.
- new yellow regions to do
- E06 (rpt, lower conc)
- E07 (rpt, lower conc)
- E08toE09
- E07toE09